
The latest news from the world of Bioplastics
World’s first plant-based medical grade face mask authorized under EUA by U.S. FDA
External source
Gorinchem, The Netherlands - PADM Medical Group of Companies (PADM Medical Canada and PADM Medical USA), has received its Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA)[1] to market PRECISION ECO™, the world’s first plant based, procedural mask with ear loops for use in healthcare and medical settings[2] in the United States of America[3].
Increasingly, governments and consumers are recognizing the environmental need to develop recyclable and compostable products. Most disposable Personal Protective Equipment (PPE) can take up to 450 years to decompose in the natural environments. Using bioplastics, such as PLA is a solution to tackle hygiene and safety needs in medical settings.
Derek Atkinson, VP of Business Development at TotalEnergies Corbion said: “We should not accept the limitations of the current way of doing things as being the only way. As we try to minimize the impact the of our products on the environment, it is these developments that help us realize these ambitions.” He continued, “as the supplier to PADM Medical Group of the high purity polylactic acid Luminy® PLA needed in the production of these groundbreaking biobased surgical masks, we are delighted to learn that PADM has succeeded in obtaining Emergency Use Authorization from the US FDA.”
The PLA procedural masks reduce the adverse impact on the environment from single use disposable face masks. Produced from sugarcane, Luminy® PLA greatly reduces the carbon footprint of this applications.
The PRECISION ECO™ Compostable/Plant Based Procedural Mask with Earloops is a USDA Certified Biobased product under the USDA BioPreferred Program with a biobased content of 82%.
“The Emergency Use Authorization on PLA facemasks is a milestone. It is a reward to our commitment to making eco-conscious products to support the health and wellness of all individuals and our planet.” said Martin Petrak, PADM Medical Group CEO.
Learn more about developing your PLA applications: https://www.totalenergies-corbion.com/applications-solutions/
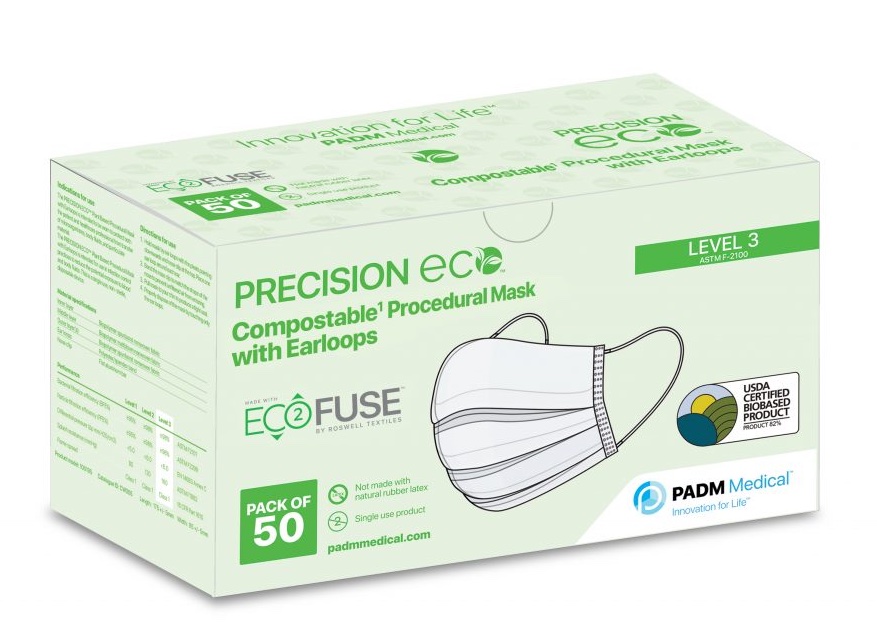
Luminy PLA based face mask with 83% USDA Biobased Preferred Certification.
Picture: PADM medical(c)
Significantly improved product lifecyle and reduced environmental impact for Luminy PLA based face masks.
Picture PADM Medical(c)
[1] The product has not been FDA cleared or approved.
[2] The product has been authorized by FDA under an EUA for use in healthcare settings by Health Care Practitioners (HCP) as PPE to provide a physical barrier to fluids and particulate materials to prevent HCP exposure to respiratory droplets and large particles during surgical mask shortages resulting from the COVID-19 pandemic.
[3] This product is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of medical devices, including alternative products used as medical devices, during the COVID-19 outbreak, under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1) unless the authorization is terminated or revoked sooner.
END PRESS RELEASE
For more information please contact:
Rui Veras
Marketing Communications Manager
M +31 629 055 522
E rui.veras@totalenergies-corbion.com
François de Bie
Senior Marketing & Supply Chain Director
M +31 611 716 895
E francois.debie@totalenergies-corbion.com





